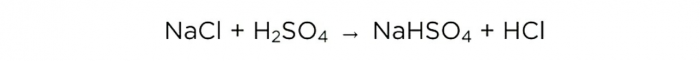Making hydrochloric acid at home
Alchemists who first produced hydrochloric acid in the 15th century called it “spiritus salis,” “spirit from salt.” At that time, this acid had magical properties: it corroded paper, dissolved metals, and poisoned people. These properties remain with it to this day, but now this acid has been sufficiently studied, and there is no magic here.
Hydrochloric acid (HCl) is a strong monobasic acid, in its pure form it is a transparent liquid. At its maximum concentration of 38% it “smoke” in air. We will receive acid with a concentration of half that.
So let's get started.
Safety precautions
Be careful when working with toxic substances!
All experiments must be carried out in a well-ventilated room or under a hood. Be sure to wear safety glasses (can be purchased at a hardware store) and gloves (if you can’t find special chemical gloves, good quality ones are suitable for washing dishes).
Baking soda must be present at the site of the experiment in order to neutralize the acid in an unforeseen situation (this will release carbon dioxide and water).
It is strictly prohibited to conduct experiments in metal containers.
Will need
To conduct the experiment we will need:
- Acid electrolyte for batteries (sold in a car store);
- Distilled water (ibid);
- Table salt (available in any kitchen);
- Baking soda (see safety precautions).
From the dishes you need:
- Glass flask;
- A vessel with sand where you can place the flask;
- Several disposable cups 200 ml;
If you have a heat-resistant flask, you can heat it under the open flame of a burner. But I still recommend using sand, in which case it will absorb acid.
You will also need a pair of plumbing angles with a diameter of 50 mm and a burner (in my case, alcohol, but I recommend using a gas one).
Stage 1 - evaporation
The electrolyte for batteries is 36% sulfuric acid (H2SO4). First we need to increase its concentration.
Pour 200 ml into the glass, that is, almost to the brim, and pour a little more than half the glass into the flask. Make a mark with a marker and add the rest.
I placed a foil reflector around the flask for more efficient heating, but later removed it because it began to melt.
Now put the flask on the burner and evaporate to the level of the previously set mark, even a little lower.
At the same time, we put gauze folded several times on the corner and secure it with an elastic band. Prepare an unsaturated soda solution and dip the end of the corner with gauze into it.
When the electrolyte begins to boil, we put a corner on the flask, it sits tightly on it. We direct the gauze end into the open window.
This is necessary if suddenly the sulfuric acid itself begins to evaporate along with the water. If you don't overheat the flask too much, this won't happen.
Burner in action:
My burner is relatively low wattage, so the evaporation took about an hour.A gas burner or electric stove would speed up this process significantly.
After completing the first stage, a little less than half of the solution should remain in the flask, that is, an acid with a concentration of about 75%. Don't forget about accuracy.
Let it cool to room temperature.
Stage 2 - calculations
Now that we have concentrated sulfuric acid, we can carry out the basic reaction, it looks like this:
But first, let's do some calculations, and at the end we will compare them with what happened in practice.
So, initially we had 200ml of electrolyte with a density of 1.27 g/cm³. Looking at the table of densities of sulfuric acid, we see that this density corresponds to a concentration of 36%. Let's calculate the volume of acid:
200ml*36%=72ml - V(H2SO4)
After we evaporated the solution, its concentration, and therefore its density, increased. We look at the same table and see that a concentration of 75% corresponds to a density of 1.67 g/cm³.
Knowing the current density (p) and volume (V) of the acid, we find out the mass:
m=p*V;
m(H2SO4)=1.67g/cm³ * 72ml=120g;
m(H2SO4)=1.67g/cm³ * 72ml=120g;
Now we remember from school chemistry:
m(H2SO4)/M(H2SO4)=m(NaCl)/M(NaCl)=m(HCl)/M(HCl),
where M is the molar mass of the substance.
where M is the molar mass of the substance.
The molar masses of H2SO4, NaCl and HCl are 98, 58.5 and 36.5 g/mol, respectively. Now we can find out how much table salt is needed and how much HCl we will get.
Namely, we need 72 g of NaCl, that’s 34 ml, let’s take it in excess - a quarter of a glass.
Great, and HCl in theory will come out to 44.7 g.
The HCl density table has a g/l column. We take from there the value for a concentration of 15% - 166.4 g/l. The volume of water required to obtain 15% HCl is 44.7/166.4≈270ml. We'll take 200ml. As a result, in theory, I will get 22% hydrochloric acid.
Stage 3 - obtaining acid
We connect the two corners as follows:
And the whole structure will look like this:
The corresponding acid will condense into a vessel labeled HCl; the volume of water in it is 200 ml. Also mark the current liquid level on this container.
We remove the corners and pour the calculated amount of salt into the flask through a funnel.
The solution turns yellow.
In order for hydrochloric acid to start releasing, you need to turn on the burner. But first, we tightly attach the corners to the flask and the receiving vessel.
When acid condenses in water, “vertical waves” are formed. Also, the solution heats up and must be cooled. For example, you can place another vessel with ice below.
The reaction proceeds relatively quickly - 20 minutes, after which you can turn off the heat. Let the last vapors of hydrochloric acid dissolve in the water, and then seal the vessel hermetically. When the flask has cooled, dilute the remaining solution with water (approximately one to one) and pour it into the sewer.
Bottom line
By the mark on the vessel we determine how much liquid has been added. For me it is ⅙, that is, 17%. This is the concentration of our hydrochloric acid. Let's compare it with the one obtained in theory.
17%/22%*100%=77% - reaction yield.
It is important to note that there is no output equal to 1, there are always losses. In my case, this is an insufficiently evaporated electrolyte. Ideally, the concentration of sulfuric acid should be 90-95%.
Let's check the resulting acid for interaction with metal.
We observe rapid evolution of hydrogen. This means that the acid is suitable for further experiments.
Variations
You can use a clean glass bottle of beer or soda as a flask, but provided that the heating is as smooth as possible. Instead of PVC corners, you can take polypropylene pipes and corners of a smaller diameter (suitable for your flask).
Once again I urge you to follow safety precautions. Happy experiences everyone!
Similar master classes
Particularly interesting
Comments (19)