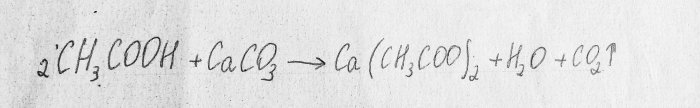DIY dry alcohol
This is what we will talk about in this article. I note that this substance only has a name in common with alcohol - we will see this later. Now let's cook it!
Will need
As chemical reagents we will use what can be easily found on the shelves.
So, to prepare solid alcohol we need:
- Acetic acid 70%, sold in grocery stores;
- Natural chalk - in garden stores;
- Medical alcohol 95% or isopropyl alcohol - in a pharmacy or car dealership, respectively.
We will also need some water and disposable (or chemical) glassware for the experiment itself, and rubber gloves. Under no circumstances should any of the above be used as food after completing the experiments!
You can use a 200 ml plastic cup as a measuring cup.
Let's start making dry alcohol
First, pour 60 ml of acetic acid and 125 ml of water into the flask.
Now add chalk to the resulting solution in small portions. Gas bubbles begin to appear, and it is important to constantly stir the solution so that they have time to burst.
The total chalk consumption will be 42 grams, but it should be added until it stops dissolving.
Consider the chemical reaction of the process:
This is a neutralization reaction.
The first substance is acetic acid. The second is calcium carbonate, which is contained in chalk. The result of this reaction is carbon dioxide, water and calcium acetate. This substance is poorly soluble in water, so during the experiment the solution may harden before your eyes:In this case, you need to add a little water and continue the experiment.
When there is no acid left in the solution, the reaction stops. Let's let him settle.
After some time, the excess chalk will settle to the bottom, since it itself is insoluble in water. Now filter the solution:I used a paper filter, you can also use a cotton pad.
The filtered solution has a yellowish tint; it is a more or less pure solution of calcium acetate. Pour a small amount into another container and add a little alcohol. A jelly-like mass begins to form:
Or a lighter shade:
Congratulations, we got solid alcohol! In fact, calcium acetate, due to the presence of the alcohol that we added, began to precipitate in the form of monohydrate; for alcohol tends to displace salts of divalent metals from solution, which is what we observe.
Now with our hands (always wearing gloves!) we give our jelly the shape of a cube or ball.
At a temperature of 160 degrees Celsius, calcium acetate decomposes, in particular, into flammable substances that promote further decomposition and support the flame.
A piece of this size can burn for up to six minutes, which is a pretty good result. And two pieces are enough to brew tea for one person. As a result of the experiment, a sufficient amount of calcium acetate was obtained, which means that it will last for a long time.
Conclusion
After production, we place the solid alcohol of the required shape in a hermetically sealed container and feel free to take it with us on a hike. And this concludes this article. Happy hiking everyone!
P.S. You can also grow colorless, needle-shaped crystals from calcium acetate, but that's a completely different story...